Life after prostate cancer
You’ve survived prostate cancer. Now, you may be living with side effects of prostate cancer treatments such as erectile dysfunction (ED) or incontinence. In your life after prostate cancer, you don’t have to live with these side effects. There are solutions available.
Even though prostate removal is considered the gold standard of care for prostate cancer, 1 in 5 men are unhappy with their functional results likely due to incontinence and sexual dysfunction, and approximately 21% of men will experience both ED and leakage after a prostatectomy.
Learn more about treatment options available to you.

Talk to a couple who’s been there
In November 2018, Tony was diagnosed with prostate cancer. He and his wife, Cece, chose to have his prostate removed. They were aware of the ED risk. After surgery, there was 100% ED along with urine leakage. He had to wear pads every day. Over a 2-year period, Tony tried many different options for ED without success. At this time, he was also changing 4 to 5 pads a day for his leakage issue on top of this. In 2021, Tony made the decision to move forward with new solutions for both his ED and incontinence – the Titan® Inflatable Penile Implant and the Virtue® Male Sling.
In his life after the implant, Tony says “Since the surgery, my confidence is back, and sexual intimacy is alive in the marriage again. Leakage is down to using one pad a day because my penile leakage was fixed during the same surgery as well. Our marriage is complete now. My wife told me the 2-year sexual drought was worth the wait. As they say in fairy tale stories… And They Lived Happily Ever After.”
Treatment options
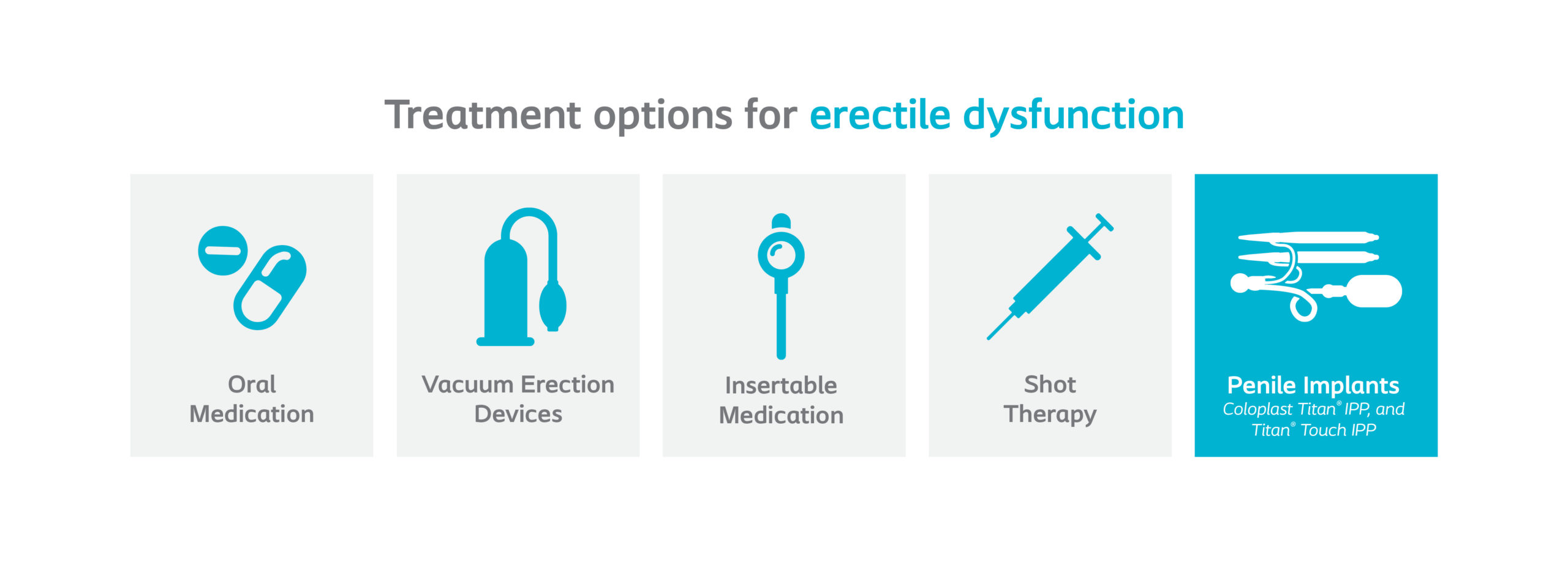 For more information on treatment options, visit: Erectile Dysfunction Treatment Options
For more information on treatment options, visit: Erectile Dysfunction Treatment Options 
Find a physician
Talking with a physician can be a benefit during your ED and/or Incontinence journey. Interested in speaking with a urologist? Locate one in our physician finder below!
Titan® and Titan® Touch Inflatable Penile Prosthesis – Important Safety Information
A penile implant, also called a penile prosthesis, is concealed entirely within the body to address erectile dysfunction (impotence). The implant requires some degree of manipulation before and after intercourse to make the penis erect or flaccid.Indications
The Titan and Titan Touch Inflatable Penile Prosthesis is indicated for male patients suffering from erectile dysfunction (impotence) who are considered to be candidates for implantation of a penile prosthesis.
Contraindications
The Titan and Titan Touch Inflatable Penile Prosthesis is contraindicated in patients who have one or more of the following: (1) Patients with an active infection present anywhere in the body, especially urinary tract or genital infection. (2) Patients with a documented sensitivity to silicone. (3) Patients with unresolved problems affecting urination, such as an elevated residual urine volume secondary to bladder outlet obstruction or neurogenic bladder. (4) Patients unwilling to undergo any further surgery for device revision.
Warnings
Implantation of the device may make latent natural erections, as well as other interventional treatment options, impossible. Men with diabetes or spinal cord injuries, as well as immunocompromised patients, may have an increased risk of infection associated with a prosthesis. Implantation of a penile prosthesis may result in penile shortening, curvature or scarring.
Precautions
Removal of an implanted prosthesis without timely reimplantation of a new prosthesis may complicate subsequent reimplantation or may make it impossible. MRI quality may be compromised if the area of interest is in the exact same area or relatively close to the position of the Titan, and Titan Touch IPP. Be sure to consult with your physician. Patients should discuss all available treatment options and their risks and benefits with their physician. Health conditions which hamper sexual activity, such as severe chest pain (angina), may prevent successful use of this device. The prosthesis should not be implanted in patients who lack the manual dexterity or strength necessary to operate the device. Trauma to the pelvic or abdominal areas, such as impact injuries associated with sports (e.g., bicycle riding), can result in damage of the implanted device and/or surrounding tissues. This damage may result in the malfunction of the device and may necessitate surgical correction, including replacement of the device. The device may be used in the presence of Peyronie’s Disease.
Potential Complications
Penile implants are surgical solutions requiring a healing period that have risks associated with surgery such as scrotal swelling, auto-inflation, discomfort, angulation/curvature, swelling (edema), device malfunction, chronic pain, difficulty with ejaculation, transient urinary retention, fever, migration, patient dissatisfaction, infection at surgical site or wound, deflation, swelling of clotted blood or clear fluid (hematoma/seroma), wound leakage, bleeding, delayed wound healing, narrowing of the opening of the foreskin (phimosis), sensory loss, cylinder malfunction, formation of thick tissue (fibrous capsule formation), over/under inflation, erosion, scrotal reddening (erythema), genital change, and inguinal hernia.
This treatment is prescribed by your physician. Discuss the treatment options with your physician to understand the risks and benefits of the various options to determine if a penile implant is right for you.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Virtue® Male Sling System – Important Safety Information
A male sling implant is concealed entirely within the body to address stress urinary incontinence (urine leakage) in men. The Coloplast Virtue Male Sling System is an implantable, suburethral support sling indicated for the treatment of male stress urinary incontinence (SUI).
It is the responsibility of the surgeon to advise the prospective patients or their representatives, prior to surgery, of the contraindications associated with the use of this product. The product is contraindicated for the following patients:
- Patients with urinary tract infections or urinary tract obstruction.
- Patients with blood coagulation disorders or prescribed anticoagulation therapy.
- Patients with blockage of urine flow (obstructive uropathy).
- Patients under the age of 18
It is the responsibility of the surgeon to advise the prospective patients or their representatives, prior to surgery, of the possible warnings associated with the use of this product.
Check with your physician on the warnings, precautions and risks associated with the use of this mesh sling.
Patients should be evaluated for the following before a sling is implanted: persistence of new onset (de novo) incontinence, good bladder function, presence of bladder neck or urethral narrowing or blockage (strictures), active urinary tract infection, and bladder muscle (detrusor) overactivity.
Patients with the following conditions should carefully consider the risks and benefits of using Virtue: blood clotting (coagulation) disorders, kidney problems (renal insufficiency) due to urinary tract obstruction and compromised immune systems or any other conditions that affect healing.
You should follow your physician’s instructions. Patients should avoid physical strain, sexual intercourse and heavy lifting for 8 weeks, and see their surgeon immediately to report any bleeding, pain, painful urination (dysuria), or signs of infection. Although rare, some infections may require the mesh to be repositioned, loosened or tightened (revised), or removed.
No undesirable effects that could be directly attributed to the polypropylene fibers/materials have been reported in the literature. As with all foreign bodies, the Virtue sling system is likely to exacerbate any existing infection. Transitory local irritation at the wound site and a foreign body response may occur. The resulting response could lead to rupture of the surgical incision (wound dehiscence), extrusion, erosion, inflammation or abnormal connection between two hollow spaces (fistula formation). The following complications are known to occur with synthetic slings: urethral erosion, infection, bladder, urethra, vessel and nerve perforation. Patients should be monitored regularly after the device has been implanted. Known risks of incontinence surgical procedures include extrusion, erosion, infection, sling migration, pain, transient or permanent retention, bladder outlet obstruction, and, continued stress urinary incontinence and persistent or new overactive bladder symptoms. The occurrence of these responses may require operative intervention with removal of part of or the complete sling.
This treatment is prescribed by your physician. This list of risks is not necessarily comprehensive. Discuss the treatment options with your physician to understand the risks and benefits of the various options to determine if a male sling is right for you.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
